Global Inferior Vena Cava (IVC) Filter Industry: Key Statistics and Insights in 2024-2032
Summary:
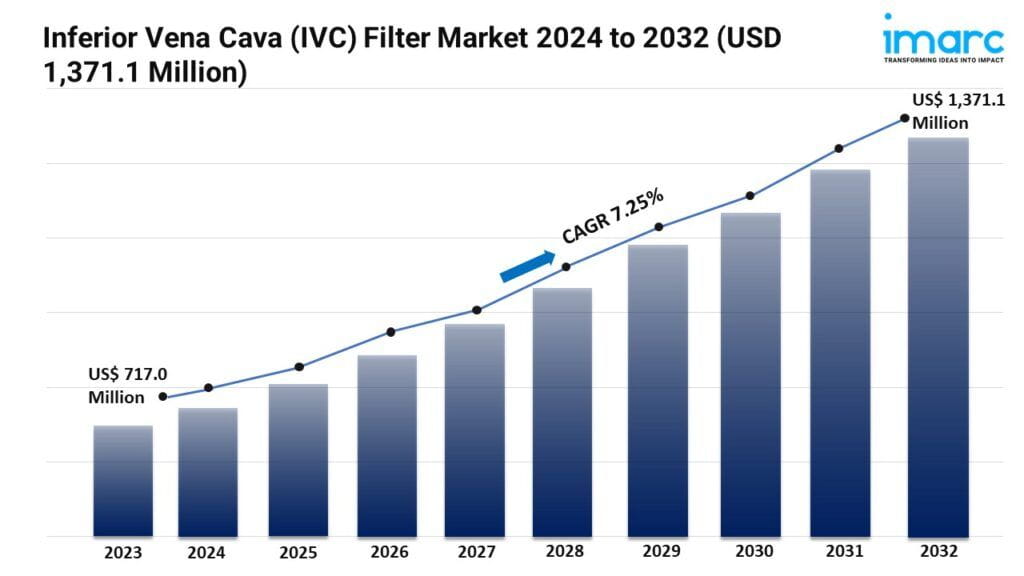
- The global inferior vena cava (ivc) filter market size reached US$ 717.0 Million in 2023.
- The market is expected to reach USD 1,371.1 Million by 2032, exhibiting a growth rate (CAGR) of 7.25% during 2024-2032.
- North America leads the market, accounting for the largest inferior vena cava ivc filter market share.
- Since they can be removed once the risk of venous thromboembolism (VTE) has passed, retrievable IVC filters are a clear market leader because of their versatility in providing short-term protection against VTE.
- Due to its compatibility with MRI treatments, which minimizes issues for patients in need of imaging diagnostics, non-ferromagnetic material makes up the largest market.
- The market is expanding due to the rising incidence of venous thromboembolism (VTE), which includes diseases like pulmonary embolism (PE) and deep vein thrombosis (DVT).
- The market is growing as a result of ongoing developments in medical technology. With improved patient safety and treatment flexibility, permanent and retrievable filters are being developed as a result of innovations in filter design.
Industry Trends and Drivers:
- Rising Incidence of Venous Thromboembolism (VTE):
The increasing prevalence of venous thromboembolism (VTE), encompassing conditions, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), is impelling the market growth. VTE incidents are common among older adults, individuals with sedentary lifestyles, and patients undergoing major surgeries. This uptick in cases necessitates effective preventive measures, particularly for those at high risk of recurring embolic events where anticoagulant therapy is unsuitable or ineffective. Inferior Vena Cava (IVC) filters, designed to trap emboli before they reach the lungs, provide a critical solution in such scenarios. The demographic shift towards an aging population further drives this need, as older individuals are more susceptible to VTE.
- Technological Advancements in Filter Design:
Continuous advancements in medical technology are propelling the market growth. Innovations in filter design are leading to the development of retrievable and permanent filters, offering enhanced patient safety and treatment flexibility. Modern IVC filters feature improved biocompatibility, reduced risk of complications, such as filter migration or fracture, and easier retrievability. These advancements cater to the specific needs of various patient demographics, ensuring a broader application range. Additionally, improvements in minimally invasive (MI) insertion techniques are making the procedure more accessible and appealing to both healthcare providers and patients. Enhanced imaging technologies are also facilitating more precise placement of IVC filters, minimizing procedural risks, and optimizing therapeutic outcomes.
- Expanding Applications in Preventive Care:
The role of IVC filters is expanding beyond traditional therapeutic uses into broader preventive care applications. Prophylactic use of IVC filters in high-risk surgical patients, trauma patients, and those with temporary contraindications to anticoagulants is gaining traction. This preventive approach aims to reduce the incidence of PE, a potentially fatal complication, especially in patients unable to receive conventional anticoagulation therapy. Clinical guidelines and recommendations are increasingly supporting the use of IVC filters in these preventive scenarios, driven by robust clinical evidence demonstrating their efficacy and safety. Furthermore, the growing focus on comprehensive patient management strategies in hospitals and healthcare facilities underscores the importance of preventive interventions.
Request for a sample copy of this report: https://www.imarcgroup.com/inferior-vena-cava-filter-market/requestsample
Inferior Vena Cava (IVC) Filter Market Report Segmentation:
By Product:

- Retrievable IVC Filter
- Permanent IVC Filter
Retrievable IVC filter exhibits a clear dominance in the market due to their flexibility in allowing temporary protection against venous thromboembolism (VTE) with the option of removal once the risk subsides.
By Material:
- Non-Ferromagnetic Material
- Ferromagnetic Materials
Non-ferromagnetic material represents the largest segment because they are compatible with MRI procedures, reducing complications for patients requiring imaging diagnostics.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America dominates the market driven by its advanced healthcare infrastructure, high incidence of VTE, and strong adoption of innovative medical technologies.
Top Inferior Vena Cava (IVC) Filter Market Leaders:
The inferior vena cava ivc filter market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- ALN Implants Chirurgicaux
- Argon Medical Devices Inc.
- B. Braun Melsungen AG
- Becton Dickinson and Company
- Braile Biomédica
- Cook Group Incorporated
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145